The effects of Water Quality in Irrigated Agriculture – By Allan Huysamen (Technical Manager – Adjuvants)
Introduction
Water is an excellent solvent for polar molecules and charged species, including simple inorganic anions and cations. Consequently, when water interacts with mineral deposits, soils, rocks or organic material, species are solubilised within the water, defining the water’s properties. The suitability of a water source for irrigation use, and its subsequent effects on soil and crop health, largely depends on the concentration and identity of dissolved inorganic ions. This needs to be understood in order to manage the water effectively.
Factors impacting water suitability
The suitability of water for irrigation depends on various factors, including salinity, sodium danger, nutrient cation concentrations, metal ion concentrations, pH, alkalinity and toxicity-inducing ions. These factors, their respective impact on water suitability and how they can be managed, require a more detailed discussion.
Salinity
The overall dissolved salts content, conveniently represented by the electrical conductivity (EC) measurement, allows for an understanding of the total concentration of dissolved ions in the water. Irrigating with water containing a very low dissolved salts content (EC < 0.4 mS.cm-1) may contribute to the leaching of ions from the topsoil, dispersal of soil particles and an ultimate reduction in water infiltration capacity. Conversely, water sources with a high dissolved salts content (EC > 0.75 mS.cm-1) will deposit surplus ions into the soil profile, resulting in a compounding effect with each irrigation cycle and as water is removed by evapotranspiration or surface evaporation. An increased ion concentration in the root zone will disturb the osmotic potential of the soil water solution and will eventually lead to a reduction in effective plant-available water. In well-draining soils, a leaching strategy can be employed to lower the soil solution salt content, by periodically applying excess water to push salts to below the root zone.
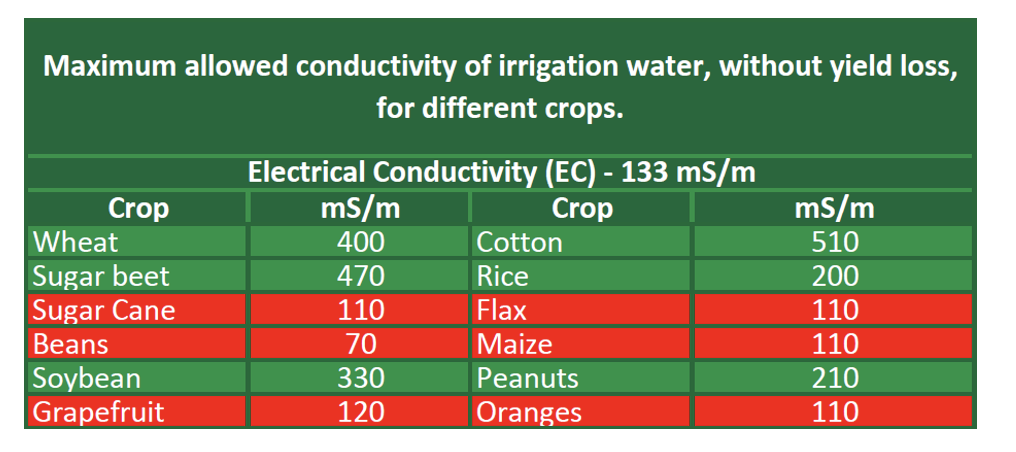
Different crops have varying salinity tolerance levels in relation to the EC of irrigation water. The use of high-salinity water should be avoided on crops with a low salinity tolerance, especially if leaching is not possible due to poor drainage or inadequate water availability. Understanding the salt-tolerance of your crops relative to the salinity of your irrigation water, as provided and displayed in the Agri Technovation ITEST™ WATER – Irrigation Report (Table 1), is therefore important to guide decisions on the use of the water source for irrigation.
Table 1: Excerpt from an ITEST™WATER – Irrigation Report, displaying the analysed EC value along with threshold EC values for various crops, above which production potential loss is expected.
Sodium Adsorption Ratio (SAR)
The SAR is the calculated value, characterising the abundance of sodium ions relative to calcium and magnesium ions in water. Water with a high SAR value indicates that sodium ions are present in a disproportionally higher concentration, which is likely to result in an increased representation of sodium on soil colloids. Apart from the nutritional effects of a high exchangeable sodium percentage, sodium dominance on soil colloids can lead to the dispersion of soil particles. This could ultimately lead to the degradation of soil structure, resulting in poor water infiltration, increased compaction and a reduction in rootzone oxygen content, thereby negatively affecting crop prosperity and productivity. The soil can however be remediated with ameliorants such as gypsum, which supply calcium cations to displace sodium from soil colloids, and sulphate anions to facilitate leaching of displaced sodium ions.
Water with a high SAR (> 10) should be avoided on fine-textured soils with a high cation exchange capacity (CEC), while even moderate SAR values (> 3) may necessitate the relatively consistent application of ameliorants to preserve soil structure.
Nutrient Cations
The Ratios and concentrations of nutritive cations such as potassium, magnesium and calcium, are also significant. A high concentration of potassium in water sources is rare and usually suggests a water source polluted with fertiliser. Potassium in high concentrations can interfere with the uptake of other ions, such as magnesium. Additionally, a disproportionally high concentration of magnesium can have a similar effect to that of sodium on the integrity of soil structure, potentially causing infiltration problems and promoting the formation of undesirable soil clods.
Metal Ions
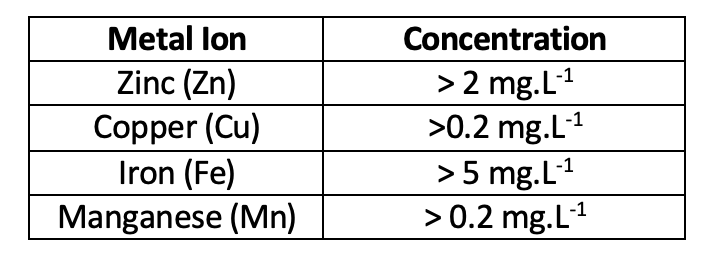
Although certain metal ions are required for plant nutrition, they can be problematic at certain concentrations and under certain conditions. Metal ions generally exhibit a greater solubility and availability for uptake under acidic conditions (pH < 7), which can present toxicity concerns based on their concentrations. Conversely, under alkaline (pH > 7) conditions, the oxidation and precipitation of metal ions are promoted, which can result in blockages of finer irrigation systems. The concentration at which the potential for toxicity under acidic conditions arises varies per metal (Table 2) and in terms of crop-specific sensitivity.1 Iron and manganese are also largely responsible for blockages of fine irrigation systems under more alkaline conditions, especially when their concentration exceeds 0.4 mg.L-1.
Table 2: Table of metal ions and the corresponding concentrations at which the ion has the potential for toxicity under acidic conditions.
The concentration of metal ions, particularly iron and manganese, can be reduced through chemical oxidation or aeration, followed by filtration or settling.
pH and Alkalinity
Water pH governs the physical state and the plant-availability of the ions solubilised in the water, although the water pH is expected to be neutralised or equilibrated to some extent as it comes into contact with the buffering soil solution. Maximum availability of the primary and secondary macronutrients is achieved around a neutral pH, whereas micronutrients largely become more available as the pH tends to 5. However, an alkaline water pH will stimulate the precipitation of carbonate salts, such as CaCO3, as well as metal ions.
The alkalinity of a water source is directly dependent on the concentration of bicarbonate and carbonate ions in the water source, defining the water’s buffering capacity. A low alkalinity (< 40 mg.L– 1 as CaCO3) will cause the water to be susceptible to pH fluctuations, which is particularly important to consider during fertigation. A high alkalinity (> 90 mg.L-1 as CaCO3) will cause the water to resist acidification, as well as promote the precipitation of CaCO3 and other carbonate salts. Irrigating with high alkalinity water can cause operational issues, including the deposition of CaCO3 in irrigation systems, eventually leading to blockages. The use of highly alkaline water has also been reported to cause nutritional disorders through interfering with the uptake and translocation of certain ions, including calcium and iron.2
High alkalinity water can be rectified through an acidification strategy, which acts to neutralise carbonate and bicarbonate species in the water and thereby reduces the alkalinity to a more favourable level. This can be achieved through the application of acidic fertilisers in the water, or by the injection of certain acids at a calculated rate.
Toxicity-Inducing Ions
Certain ions will have a toxic effect on crops when the ions are present above their respective threshold concentrations. This will eventually lead to loss of production potential. Ions that can cause a toxic effect include boron and chloride, which are both necessary for plant nutrition at lower concentrations. In excess and as concentrations in the irrigation water increase, they will eventually cause chlorosis, necrosis, and loss of photosynthetic potential. The severity of the toxicity response for a given concentration of chloride or boron is crop-specific, the most sensitive crops generally being fruit and nut crops. Removal of chloride and boron from irrigation water is usually not feasible, leaving mixing of water sources as one of the only practical solutions for lowering the concentration of either ions in irrigation water.
Having knowledge of the concentration of toxicity-inducing ions in your irrigation water sources and understanding the tolerance of your crops towards these ions is extremely important for water source selection and management. Alongside the analysed concentrations provided in the Agri Technovation ITEST™WATER – Irrigation Report, crop-specific sensitivities are highlighted to allow foresight into toxicity concerns (Figure 1).
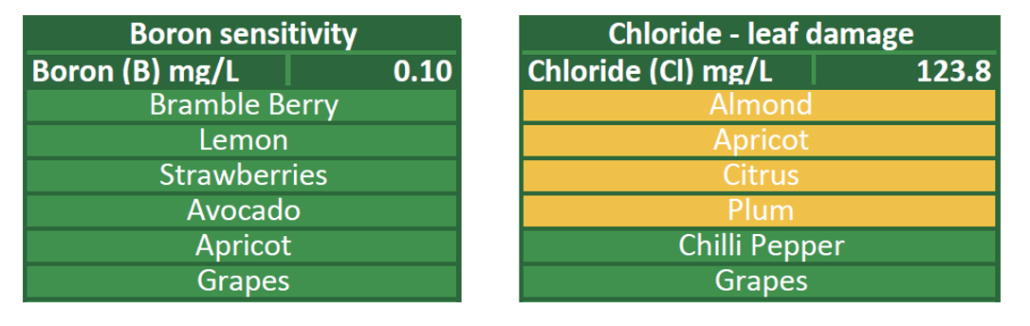
Figure 1: Excerpt from an ITEST™WATER – Irrigation Analysis Report, displaying the analysed chloride and boron concentrations and highlighting crop sensitivity to the analysed concentrations.
A Solution : ITEST™WATER – Irrigation Analysis
Agri Technovation’s ITEST™WATER – Irrigation analysis service aims to provide the most important information necessary to assess the suitability of a water source for irrigation use. Analysis data is displayed in a graphical format to allow for ease of interpretation (Figure 2), while also taking crop- specific sensitivities into account where applicable. This allows for relatively rapid identification of potential problems and comparison between various water sources, as well as highlighting where the implementation of management strategies may be necessary. Regular analysis of a single water source is also important for monitoring changes in water quality over time, as changes occur in the short- term due to drought or excessive rainfall, and in the long-term due to anthropogenic factors and a changing climate.
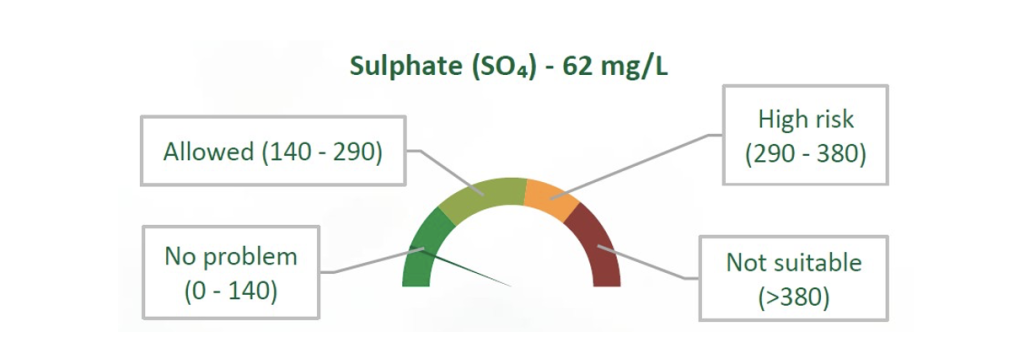
Figure 2: A graph as provided in the ITEST™WATER – Irrigation Report, displaying the analysed sulphate concentration and associated bounds for suitability.
References
- S. Ayers and D.W. Westcot, Water Quality for Agriculture, FAO, Rome, 1985.
- Shahabi, M.J. Malakouti and E. Fallahi, J. Plant Nutr., 2005, 28, 1663-1678.

